My son was diagnosed with Keratoconus in December 2007 just as he turned 11 years old. In June 2008, we went to Price Vision in Indianapolis, IN to see about the CXL study and whether he would qualify with an age exception to the study. Upon examination of his eye, they determined his cornea was already too thin. This was only 6 months after diagnosis.
Carson also has Down syndrome. Because of this as well as him just being a kid, he would never be able to sit still for the CXL procedure, even with sedation and local anesthesia. Instead, he would need general anesthesia to ensure no movement of the eye. For this reason, the right eye was not even considered and we went home very depressed.
For our kids with Down syndrome, there is no contact option for visual clarity, as for other Keratoconus patients. Any visual correction must be done with glasses. But even eye exams aren’t like they are for the typical population. You can get a general prescription, but never the fine tuning like for typical people. So even the correction isn’t that great. Even with his glasses on, right now Carson has to be about 2 inches from any books he reads.
That really impacts his education. How can you get any fluency in reading when all you can focus on is one word at a time? It really breaks my heart as his mom to see him so affected by this horrible Keratoconus, especially when I am powerless to stop this dreadful condition. The other day in the store, he was about 20 feet from me in a direct line of sight and couldn’t find me without me talking. Even now I have tears in my eyes just thinking and writing about how limited his vision is.
For us, a cornea transplant is huge. The actual surgery doesn’t concern me. After all, about 40,000 cornea transplants are performed each year. What does concern me are the days, months and years afterwards. With limited verbal communication, how will Carson ever be able to convey the early signs of a transplant rejection, when it might still be salvaged. Furthermore, would he even recognize the early signs of rejection or a popped stitch? And how would I keep him from ever rubbing his eye, even if he wore a clear eye guard for months? I know it can be done (and it must, for we have no choice with the left eye now), but it’s really mind-boggling for me as his mom.
In October 2008, Carson developed acute hydrops in his left eye. It’s a very painful condition where the inner membranes of the cornea separate. His cornea specialist had said this was a possibility, I just didn’t expect it so soon. His specialist also said he’d never seen a case of Keratoconus progress so fast. Great!
It’s so frustrating as his parent having his eyes deteriorate so fast while there’s this great procedure out there that could stop the progression and delay or even prevent a cornea transplant. We just can’t get to it. Not only is he too young for the trial, he would need general anesthesia and that’s just not available in the eye doctor’s office.
If CXL were already approved by the FDA, we could probably fairly easily and in a timely manner find a cornea specialist in the US that would perform the procedure in a hospital setting so that general anesthesia could also be used. That’s why FDA approval of CXL is so important to us.
There’s no way CXL will get FDA approval in time to stop Carson’s Keratoconus. But I am advocating for a “Fast Track” approval of CXL for all the other people out there that don’t have the time to wait. The Food and Drug Administration Modernization Act of 1997 (FDAMA, P.L. 105-115) directed the Secretary to create a mechanism whereby FDA could designate as “Fast Track” certain products that met two criteria. First, the product must concern a serious or life-threatening condition; second, it has to have the potential to address an unmet medical need. For more info on FDA “Fast Track” approval, go to ttp://assets.opencrs.com/rpts/RS22814_20080221.pdf. I think CXL meets that criteria.
Please help us get a “Fast Track” approval for CXL by writing to your US legislators. If applicable, tell them your story and how CXL would affect you. If you don’t have a story, advocate on behalf of all of us that do.
skip to main |
skip to sidebar
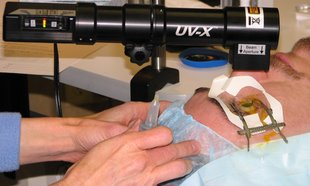
CXL is a procedure that involves administering riboflavin and UVA in carefully selected parameters that strengthen the front layers of the cornea and avoid damage to the back of the eye.
Click on below link to Watch in HD on large screen
Get an email everytime we post with new update.
CXL Procedure

CXL is a procedure that involves administering riboflavin and UVA in carefully selected parameters that strengthen the front layers of the cornea and avoid damage to the back of the eye.
Keratoconus and CXL links and articles
- Nick Richards blog
- Video Clip about CXL
- Collagen Crosslinking with Riboflavin as a Photosynthesizer
- ClinicalTrial.gov (U.S. CXL study sites)
- One of a Kind Eye Treatment
- Mom Always Said, "Don't Rub Your Eyes"
- National Keratoconus Foundation
- Cornea Research Foundation of America
- The Global Keratoconus Foundation
- Keratoconus on Wikipedia
Blog Archive
About Us
- Janis "Widget" (Marietta, GA) and Connie (Mason, OH)
- We are each moms of incredible sons with Down syndrome who have also both been diagnosed with Keratoconus, one at age 25 and one at age 12. It progressed very rapidly in both our sons, going from mild to advanced in less than a year. CXL seemed like a miracle for us. This blog is to bring awareness of this wonderful treatment of Keratoconus and to encourage the FDA to "fast track" the approval of CXL.

