Tuesday, February 24, 2009
Sunday, February 22, 2009
Why CXL is so important to me (by Connie)
My son was diagnosed with Keratoconus in December 2007 just as he turned 11 years old. In June 2008, we went to Price Vision in Indianapolis, IN to see about the CXL study and whether he would qualify with an age exception to the study. Upon examination of his eye, they determined his cornea was already too thin. This was only 6 months after diagnosis.
Carson also has Down syndrome. Because of this as well as him just being a kid, he would never be able to sit still for the CXL procedure, even with sedation and local anesthesia. Instead, he would need general anesthesia to ensure no movement of the eye. For this reason, the right eye was not even considered and we went home very depressed.
For our kids with Down syndrome, there is no contact option for visual clarity, as for other Keratoconus patients. Any visual correction must be done with glasses. But even eye exams aren’t like they are for the typical population. You can get a general prescription, but never the fine tuning like for typical people. So even the correction isn’t that great. Even with his glasses on, right now Carson has to be about 2 inches from any books he reads.
That really impacts his education. How can you get any fluency in reading when all you can focus on is one word at a time? It really breaks my heart as his mom to see him so affected by this horrible Keratoconus, especially when I am powerless to stop this dreadful condition. The other day in the store, he was about 20 feet from me in a direct line of sight and couldn’t find me without me talking. Even now I have tears in my eyes just thinking and writing about how limited his vision is.
For us, a cornea transplant is huge. The actual surgery doesn’t concern me. After all, about 40,000 cornea transplants are performed each year. What does concern me are the days, months and years afterwards. With limited verbal communication, how will Carson ever be able to convey the early signs of a transplant rejection, when it might still be salvaged. Furthermore, would he even recognize the early signs of rejection or a popped stitch? And how would I keep him from ever rubbing his eye, even if he wore a clear eye guard for months? I know it can be done (and it must, for we have no choice with the left eye now), but it’s really mind-boggling for me as his mom.
In October 2008, Carson developed acute hydrops in his left eye. It’s a very painful condition where the inner membranes of the cornea separate. His cornea specialist had said this was a possibility, I just didn’t expect it so soon. His specialist also said he’d never seen a case of Keratoconus progress so fast. Great!
It’s so frustrating as his parent having his eyes deteriorate so fast while there’s this great procedure out there that could stop the progression and delay or even prevent a cornea transplant. We just can’t get to it. Not only is he too young for the trial, he would need general anesthesia and that’s just not available in the eye doctor’s office.
If CXL were already approved by the FDA, we could probably fairly easily and in a timely manner find a cornea specialist in the US that would perform the procedure in a hospital setting so that general anesthesia could also be used. That’s why FDA approval of CXL is so important to us.
There’s no way CXL will get FDA approval in time to stop Carson’s Keratoconus. But I am advocating for a “Fast Track” approval of CXL for all the other people out there that don’t have the time to wait. The Food and Drug Administration Modernization Act of 1997 (FDAMA, P.L. 105-115) directed the Secretary to create a mechanism whereby FDA could designate as “Fast Track” certain products that met two criteria. First, the product must concern a serious or life-threatening condition; second, it has to have the potential to address an unmet medical need. For more info on FDA “Fast Track” approval, go to ttp://assets.opencrs.com/rpts/RS22814_20080221.pdf. I think CXL meets that criteria.
Please help us get a “Fast Track” approval for CXL by writing to your US legislators. If applicable, tell them your story and how CXL would affect you. If you don’t have a story, advocate on behalf of all of us that do.
Carson also has Down syndrome. Because of this as well as him just being a kid, he would never be able to sit still for the CXL procedure, even with sedation and local anesthesia. Instead, he would need general anesthesia to ensure no movement of the eye. For this reason, the right eye was not even considered and we went home very depressed.
For our kids with Down syndrome, there is no contact option for visual clarity, as for other Keratoconus patients. Any visual correction must be done with glasses. But even eye exams aren’t like they are for the typical population. You can get a general prescription, but never the fine tuning like for typical people. So even the correction isn’t that great. Even with his glasses on, right now Carson has to be about 2 inches from any books he reads.
That really impacts his education. How can you get any fluency in reading when all you can focus on is one word at a time? It really breaks my heart as his mom to see him so affected by this horrible Keratoconus, especially when I am powerless to stop this dreadful condition. The other day in the store, he was about 20 feet from me in a direct line of sight and couldn’t find me without me talking. Even now I have tears in my eyes just thinking and writing about how limited his vision is.
For us, a cornea transplant is huge. The actual surgery doesn’t concern me. After all, about 40,000 cornea transplants are performed each year. What does concern me are the days, months and years afterwards. With limited verbal communication, how will Carson ever be able to convey the early signs of a transplant rejection, when it might still be salvaged. Furthermore, would he even recognize the early signs of rejection or a popped stitch? And how would I keep him from ever rubbing his eye, even if he wore a clear eye guard for months? I know it can be done (and it must, for we have no choice with the left eye now), but it’s really mind-boggling for me as his mom.
In October 2008, Carson developed acute hydrops in his left eye. It’s a very painful condition where the inner membranes of the cornea separate. His cornea specialist had said this was a possibility, I just didn’t expect it so soon. His specialist also said he’d never seen a case of Keratoconus progress so fast. Great!
It’s so frustrating as his parent having his eyes deteriorate so fast while there’s this great procedure out there that could stop the progression and delay or even prevent a cornea transplant. We just can’t get to it. Not only is he too young for the trial, he would need general anesthesia and that’s just not available in the eye doctor’s office.
If CXL were already approved by the FDA, we could probably fairly easily and in a timely manner find a cornea specialist in the US that would perform the procedure in a hospital setting so that general anesthesia could also be used. That’s why FDA approval of CXL is so important to us.
There’s no way CXL will get FDA approval in time to stop Carson’s Keratoconus. But I am advocating for a “Fast Track” approval of CXL for all the other people out there that don’t have the time to wait. The Food and Drug Administration Modernization Act of 1997 (FDAMA, P.L. 105-115) directed the Secretary to create a mechanism whereby FDA could designate as “Fast Track” certain products that met two criteria. First, the product must concern a serious or life-threatening condition; second, it has to have the potential to address an unmet medical need. For more info on FDA “Fast Track” approval, go to ttp://assets.opencrs.com/rpts/RS22814_20080221.pdf. I think CXL meets that criteria.
Please help us get a “Fast Track” approval for CXL by writing to your US legislators. If applicable, tell them your story and how CXL would affect you. If you don’t have a story, advocate on behalf of all of us that do.
What is Cornea Cross-linking (CXL)?
Collagen cross-linking is a relatively new treatment for Keratoconus that uses a photosensitizing agent, riboflavin (vitamin B2) & ultraviolet light (UVA, 365nm) exposure. It was developed about ten years ago in Germany by Dr. Theo Seiler. Since then, many countries have approved the CXL procedure for treatment of Keratoconus. CXL is currently in Phase II FDA trials in the US (http://clinicaltrials.gov/ct2/show/NCT00647699).
In extensive experimental studies (including biomechanical stress & strain measurements) researchers have demonstrated a significant increase in corneal rigidity / stiffness after collagen cross-linking using this riboflavin/UVA treatment. The 3 & 5 year results of Dresden clinical study in human eyes has shown the stop of progression of Keratoconus in all treated eyes. (Wollensak G. Crosslinking treatment of progressive keratoconus: New Hope. Current Opinion in Ophthalmology 2006; 17: 356 – 360)
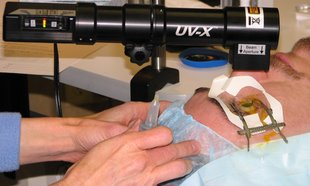
The treatment is performed under topical anesthesia. The skin (epithelium) of the surface of the cornea is partially scratched, followed by application of Riboflavin eye drops for 30 minutes. The eye is then exposed to UVA light for 30 minutes. After the treatment, antibiotic ointment is applied and an eye-pad is worn overnight until the next day when the surface of the eye has healed. Oral analgesics are required for the first 1 -2 days.
Collagen cross-linking treatment is not a cure for keratoconus. Instead, it aims to halt the progression of Keratoconus. This is important to understand. Patients will continue to wear glasses or contact lenses (although a change in the prescription may be required) following the cross-linking treatment. The main objective of CXL is to stop the progression of Keratoconus, thus preventing further deterioration in vision and the need for cornea transplant.
In advanced Keratoconus where the corneal thickness is below 350 microns, CXL may not be performed because of possible damage by the UVA light to other parts of the eye.
In extensive experimental studies (including biomechanical stress & strain measurements) researchers have demonstrated a significant increase in corneal rigidity / stiffness after collagen cross-linking using this riboflavin/UVA treatment. The 3 & 5 year results of Dresden clinical study in human eyes has shown the stop of progression of Keratoconus in all treated eyes. (Wollensak G. Crosslinking treatment of progressive keratoconus: New Hope. Current Opinion in Ophthalmology 2006; 17: 356 – 360)
The treatment is performed under topical anesthesia. The skin (epithelium) of the surface of the cornea is partially scratched, followed by application of Riboflavin eye drops for 30 minutes. The eye is then exposed to UVA light for 30 minutes. After the treatment, antibiotic ointment is applied and an eye-pad is worn overnight until the next day when the surface of the eye has healed. Oral analgesics are required for the first 1 -2 days.
Collagen cross-linking treatment is not a cure for keratoconus. Instead, it aims to halt the progression of Keratoconus. This is important to understand. Patients will continue to wear glasses or contact lenses (although a change in the prescription may be required) following the cross-linking treatment. The main objective of CXL is to stop the progression of Keratoconus, thus preventing further deterioration in vision and the need for cornea transplant.
In advanced Keratoconus where the corneal thickness is below 350 microns, CXL may not be performed because of possible damage by the UVA light to other parts of the eye.
What is Keratoconus?
Keratoconus (kehr-uh-toh-KOH-nus) is the most common corneal dystrophy that leads to severe visual impairment. It affects 1 in every 2000 people and is 15% more common in individuals with Down syndrome. Although not a common known eye disorder, it is by no means rare, and it is becoming more common due to the use of modern diagnostic equipment.
Keratoconus is a condition in which the normally round, dome-shaped cornea (front window of the eye) thins and becomes distorted and irregular. A cone-like bulge develops, resulting in significant visual distortion. The apex of the cone is usually displaced outwards and downwards and in the line of sight, creating irregular astigmatism.
Keratoconus is typically diagnosed in the late teens or early 20's, although it is occassionally diagnosed in young children as well as people in their 30's or 40's. It us usually slow to progress, taking 10 to 20 years, but sometimes it is very rapidly progressing going to an advanced stage in as short as 6 to 12 months from diagnosis. In our experiences, this seems to be a more common occurrance for individuals with Down syndrome. Also, At this time, there is no known cause, but studies have shown that genes on chromosomes 21, 17 and 13 may play a role. In addition, eye rubbing has been suggested to be a cause as well.
Until recently, there has been no treatment that stopped the progression of Keratoconus and instead the symptoms were treated keratoconus is treated through eyeglasses, hard contact lenses, and a newer treatment, INTACS plastic rings inserted into the mid layer of the cornea to flatten it, changing the shape and location of the cone. Again, these just corrected the distorted vision of Keratoconus patients, but did not stop the progression of the disease.
In 15-20% of the cases, cornea transplant surgery is necessary. When vision can no longer be corrected with contacts or the patient can no longer tolerate the uncomfortable contacts, a cornea transplant is the next option. Cornea transplants are the most common type of solid tissue transplant, and Keratoconus is the major cause of cornea transplantation in the Western world.
Maybe this "treatment" of Keratoconus is changing, so that rather than treat the symptoms (but still have the disease progress), the disease can actually be treated. Approximately 10 years ago, Dr. Theo Seiler developed a procedure called Corneal Cross-linking (CXL).
Keratoconus is a condition in which the normally round, dome-shaped cornea (front window of the eye) thins and becomes distorted and irregular. A cone-like bulge develops, resulting in significant visual distortion. The apex of the cone is usually displaced outwards and downwards and in the line of sight, creating irregular astigmatism.
Keratoconus is typically diagnosed in the late teens or early 20's, although it is occassionally diagnosed in young children as well as people in their 30's or 40's. It us usually slow to progress, taking 10 to 20 years, but sometimes it is very rapidly progressing going to an advanced stage in as short as 6 to 12 months from diagnosis. In our experiences, this seems to be a more common occurrance for individuals with Down syndrome. Also, At this time, there is no known cause, but studies have shown that genes on chromosomes 21, 17 and 13 may play a role. In addition, eye rubbing has been suggested to be a cause as well.
Until recently, there has been no treatment that stopped the progression of Keratoconus and instead the symptoms were treated keratoconus is treated through eyeglasses, hard contact lenses, and a newer treatment, INTACS plastic rings inserted into the mid layer of the cornea to flatten it, changing the shape and location of the cone. Again, these just corrected the distorted vision of Keratoconus patients, but did not stop the progression of the disease.
In 15-20% of the cases, cornea transplant surgery is necessary. When vision can no longer be corrected with contacts or the patient can no longer tolerate the uncomfortable contacts, a cornea transplant is the next option. Cornea transplants are the most common type of solid tissue transplant, and Keratoconus is the major cause of cornea transplantation in the Western world.
Maybe this "treatment" of Keratoconus is changing, so that rather than treat the symptoms (but still have the disease progress), the disease can actually be treated. Approximately 10 years ago, Dr. Theo Seiler developed a procedure called Corneal Cross-linking (CXL).
Subscribe to:
Posts (Atom)